Streamline® OCT System
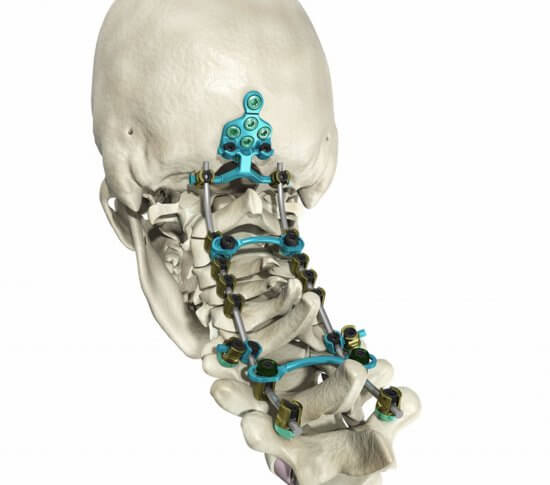
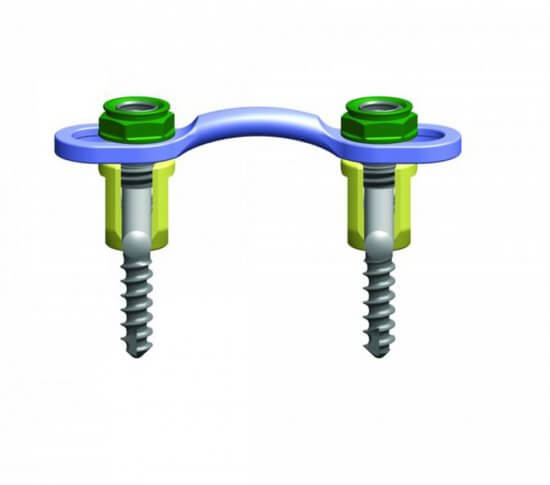
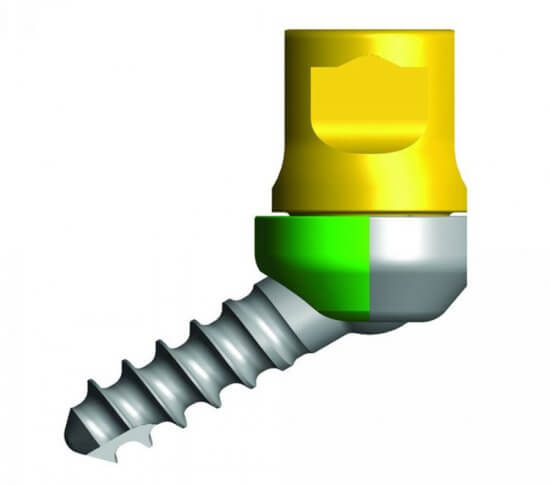
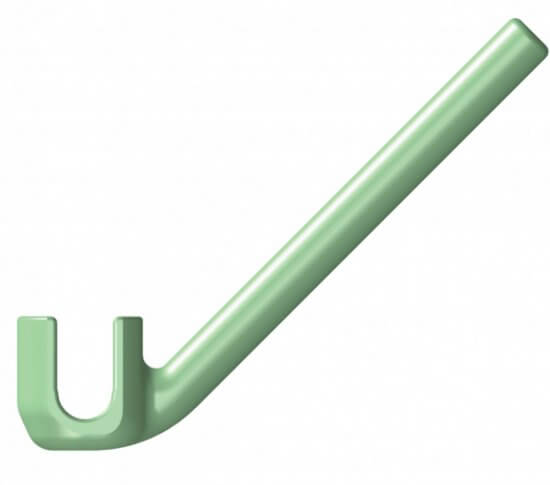
The Streamline® OCT (Occipito-Cervico-Thoracic) System allows a rigid construct to be created in the occipito-cervico-thoracic spine by offering a broad range of implants. These implants provide the ability to tailor treatment to a specific patient for a more efficient, streamlined surgical experience.
Regulatory approvals vary by country. Therefore, we kindly ask you to contact the distributor in your region regarding availability of specific products, implants and / or instrumentation in your region.